July 2023 Regulatory Update
A new test standard, which is applicable to textile and textile products, was published in May 2023, ISO 4484-3:2023 Textiles and textile products — Microplastics from textile sources — Part 3: Measurement of collected material mass released from textile end products by domestic washing method.
Read MoreA new test standard for textiles and textile products was published in May 2023. The standard is ISO 4484-3:2023 Textiles and textile products - Microplastics from textile sources — Part 3: Measurement of collected material mass released from textile end products by domestic washing method.
Daily washing of textile end products generates fiber fragments which are discharged from washing machines. The purpose of this test method is to collect materials including fiber fragments which are discharged from domestic washing machines through the washing process. This test standard applies to textile end products (including consumer textile products, such as clothing made of fleece, shirts, trousers, blouses, etc.) and home textile end products (such as, blankets, rugs, curtains, etc.) which are composed of all fibers such as natural fibers, and man-made fibers, including a mixture of the fibers that can be subjected to washing in a domestic washing machine.
This test standard does not apply to fabrics and cut textile products, nor does it cover the testing for washing machines and detergents.
The State of Maine launched a law (LD 217) amending the existing statute – “Products Containing PFAS” (38 M.R.S. § 1614). This amendment law postpones the notification deadline and gives more options on reporting.
Read MoreOn 8 June 2023, the Governor of Maine signed the bill (LD 217) into law by amending the existing statute – “Products Containing PFAS” (38 M.R.S. § 1614). The original statute requires manufacturers to report to the Department of Environmental Protection (DEP) if the products contain intentionally added perfluoroalkyl and polyfluoroalkyl substances (PFAS), beginning 1 January 2023. The amendment law postpones the reporting start date until 1 January 2025.
This amendment law provides several options on how to report PFAS cases to the DEP:
- Reporting of PFAS by CAS number
- Reporting of PFAS by a description approved by the DEP if CAS number is absent
- Reporting as exact quantity of each PFAS compound
- Reporting as the amount of Total Organic Fluorine (TOF) determined by analytical testing if exact quantity of PFAS is not available
- Reporting based on supplier information
It is interesting to note that exemptions have been introduced in the amendment law. Manufacturers employing 25 or less people are exempt from the reporting requirements. Additionally, used products or product components are exempted from the law.
In May 2023, Washington State adopted the rules for safer products priority chemical restrictions and reporting (Chapter 173-133 WAC – Safer Products Restrictions and Reporting). It is related to the work of Washington State’s Department of Ecology (DoE) under the Safer Products for Washington Program (Toxic Pollution Law, Chapter 70A.350 RCW) which is a law designed to reduce toxic chemicals in consumer products in order to protect people, the environment and sensitive populations and species. This new adopted ruling is the first cycle working of DoE where certain consumer products containing priority chemicals are restricted and reporting is required. The first restriction and reporting deadline will be applied beginning 1 January 2024 and 31 January 2025, respectively.
Read MoreOn 31 May 2023, Washington State adopted the rule for safer products priority chemical restrictions and reporting (Chapter 173-133 WAC – Safer Products Restrictions and Reporting). It is related to the work of Washington State’s Department of Ecology (DoE) under the Safer Products for Washington Program (Toxic Pollution Law, Chapter 70A.350 RCW) which is a law designed to reduce toxic chemicals in consumer products in order to protect people, the environment and sensitive populations and species. As such, the safer products program contains a process of designating priority chemicals, identifying priority consumer products, determining potential regulatory actions and also rulemaking.
The new adopted ruling is the first cycle working of the DoE where certain consumer products containing priority chemicals are restricted and reporting is required. It aims at reducing toxic chemicals by establishing restrictions and improving the transparency of product ingredients. Priority chemicals include perfluoroalkyl and polyfluoroalkyl substances (PFAS), ortho-phthalates, flame retardants, alkylphenol ethoxylates and bisphenols. The priority products covered include various products such as aftermarket stain- and water resistance treatments, carpet and rugs, leather and textile finishings, vinyl flooring, personal care product fragrances, electric and electronic products, recreational polyurethane foam, laundry detergent, food and drink can linings, and thermal paper.
The DoE has introduced a concept of a rebuttable presumption in this new adopted ruling. When the regulated products are tested for compliance and the priority chemicals are found at or above the restricted level, the DoE will presume that these chemicals are intentionally added in the products. The manufacturers can rebut this presumption by providing a statement to the DoE including supporting information that the detected chemicals are not intentionally added.
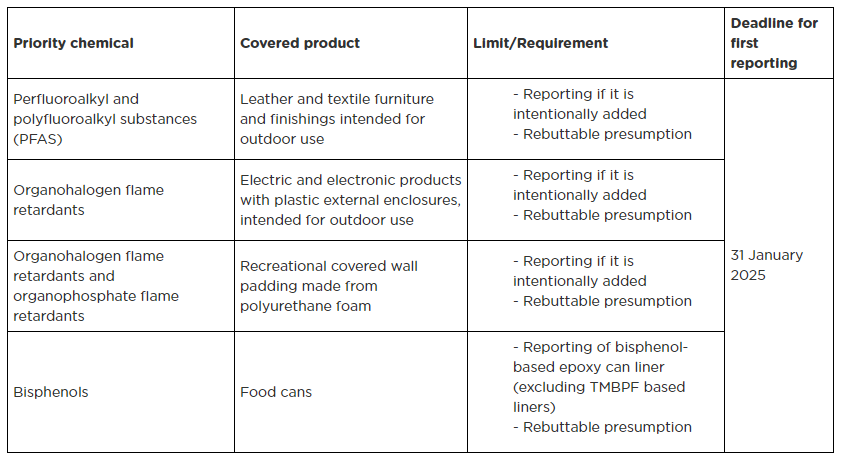
The earliest restriction and reporting date starts on 1 January 2024. The manufacturers (reporting parties) must submit the report to the DoE via the High Priority Chemicals Data System (HPCDS) by 31 January 2025; the information should be submitted annually. The reporting parties may submit a notification to the DoE when the priority consumer products no longer contain any intentionally added priority chemicals. Details of the priority chemicals and covered products under the new adopted rule of restriction and reporting are listed in below table 1 and table 2, respectively.
Table 1. Restriction requirement of priority chemicals in consumer products
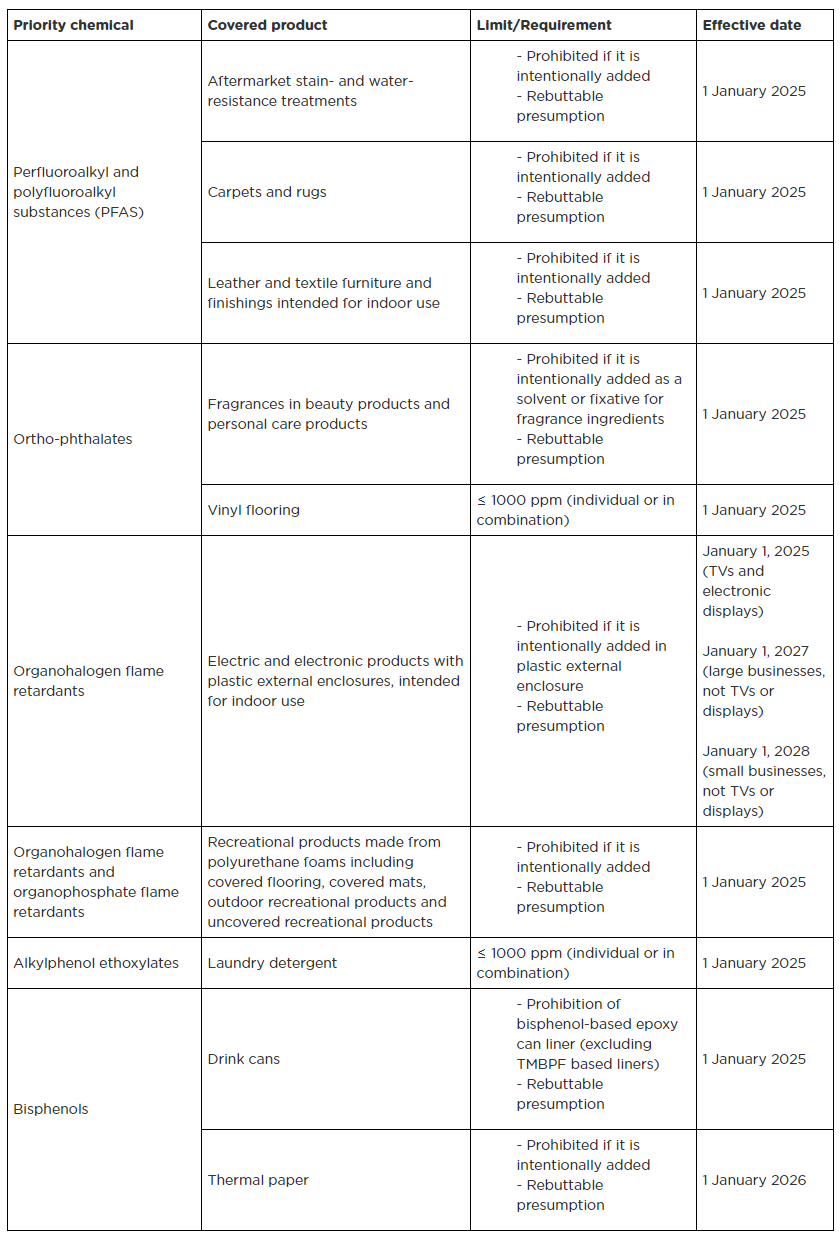
Table 2. Reporting requirement of priority chemicals in consumer products
The requirements of the old Carriages and Strollers Regulations (SOR/2016-167) were introduced in 1985 and they have not been changed substantively since that time. On 7 June 2023, Health Canada published a new Regulation for Carriages and Strollers (SOR/2023-101) in the Canada Gazette to repeal and replace the old carriages and strollers regulations.
Five categories are addressed under the new regulations which include:
- Mechanical Requirements
- Applied Coating Materials Requirements
- Toxicological Requirements
- Phthalates Requirements
- Information and Warning Requirements
The effective date for the new regulations was 7 June 2023.
Read MoreThe requirements of the Carriages and Strollers Regulations (SOR/2016-167) were originally introduced in 1985 and have had minimal changes since that time. The new regulations implemented to repeal and replace these initial regulations were published in the Canada Gazette on 7 June 2023 as SOR/2023-101. As per the new regulations, carriages and strollers are defined as below:
Stroller-means a wheeled vehicle that is designed to transport a child in a seated position and includes a wheeled vehicle that is designed to be converted to function as a stroller and has been so converted.
Carriage-means a wheeled vehicle that is designed to transport a child in a reclined or horizontal position and includes a wheeled vehicle that is designed to be converted to function as a carriage and has been so converted.
A brief introduction of the requirements of the new regulations is as below:
Mechanical Requirement
The new regulations require compliance with either:
- The requirements from ASTM F833 (excluding those set out in sections 5.3, 5.12, 8 and 9, incorporated by ambulatory reference); or
- The requirements from ISO 31110 (excluding those set out in sections 6, 7 and 10, incorporated by ambulatory reference), and, in addition, the entrapment requirements from ASTM F833 sections 6.8 and 6.10.
Applied Coating Materials Requirement
The new regulations directly include requirements consistent with section 23 of the Toys Regulations (SOR/2011-17).
Toxicological Requirement
The new regulations incorporate by reference the toxicological requirements set out in sections 22 and 25 of the Toys Regulations.
Phthalates Requirement
The new regulations address phthalates content for vinyl parts of a carriage or stroller in a manner consistent with the Phthalates Regulations (SOR/2016-188).
Information and Warning Requirement
Information and warning requirements under the new regulations include:
- Information that must be printed on carriages or strollers;
- Information that may be printed on carriages or strollers or that may accompany them;
- Information that must be printed on packaging;
- Warnings that must be printed on carriages and strollers;
- Warnings that may be printed on carriages or strollers or that may accompany them; and
- Warnings that must be printed on packaging.
A transitional provision is included in the new regulation that allows carriages and strollers that are compliant to the repealed regulations to continue to be manufactured, imported, advertised, or sold in Canada for 180 days after the new regulations come into force.
The new regulations came into force on 7 June 2023.
Canada: Health Canada has Launched a Public Consultation for the Intention to Revise the Toys Regulation
Health Canada has launched the public consultation for the intention to revise the Toys Regulation. Any interested party can provide feedback by completing the questionnaire on the Health Canada website. Comments will be accepted until 21 September 2023.
On 23 June 2023, Health Canada launched the public consultation for the intention to revise the Toys Regulation (SOR/2011-17). Any party that is interested in safety of toys is urged to provide feedback by completing the questionnaire on the Health Canada website. The website is open for public comment until 21 September 2023.
The new proposal seeks to revise seven items associated with chemical and toxicity requirements in the Toys Regulation including:
- Addition of non-human sources of toxicity data for a more comprehensive assessment of the risk exposure of toxic substances in toys
- Modification of requirement for corrosive, irritant or sensitizing substances by using weight of evidence approach
- Modification to move to testing of all toys for the presence of boric acid and salts of boric acid
- Expansion of the requirements for migration of certain elements in toys and modify the total limit of mercury
- Adoption of extra requirements for finger paints as stated in ISO 8124-7 – Safety of Toys Part 7
- Addition of requirements by incorporation of ASTM F963 Standard Consumer Safety Specification for Toy Safety by ambulatory reference for liquids, pastes, putties, gels, powders, and items of avian feather origin
- Inclusion of an ambulatory incorporation by reference with ISO 8124-11 for chemical toy (sets) other than experimental sets
A 180-day transition period is being proposed by Health Canada for future changes to documents incorporated by ambulatory reference. Regulated parties will benefit from clarity surrounding when they must comply with new editions of reference documents.
In the US, when hazards are identified in consumer products, they will be recalled and published in the Consumer Product Safety Commission (CPSC) Recent Recalls on the CPSC website, which is updated daily. The US recalls from 01 June 2023 to 30 June 2023 are summarized below:
Read More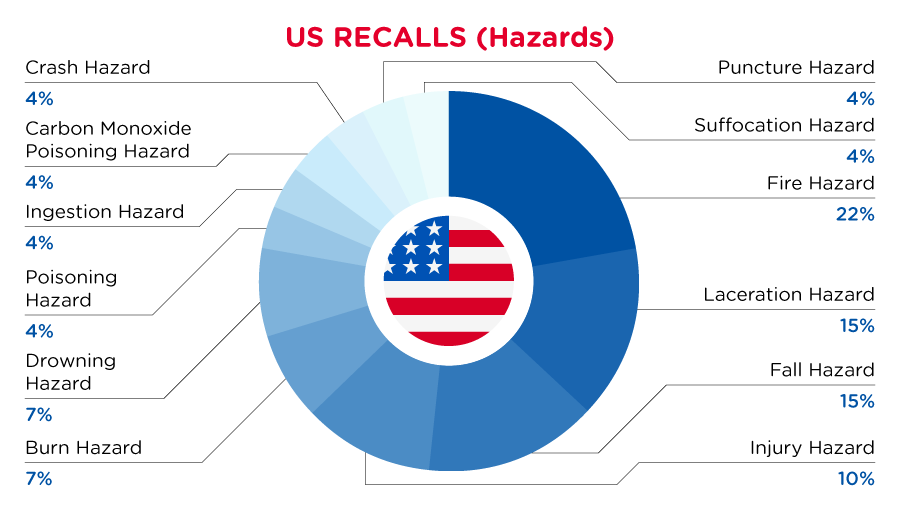
| Hazards | Frequency |
| Fire Hazard | 6 |
| Laceration Hazard | 4 |
| Fall Hazard | 4 |
| Injury Hazard | 3 |
| Burn Hazard | 2 |
| Drowning Hazard | 2 |
| Poisoning Hazard | 1 |
| Ingestion Hazard | 1 |
| Carbon Monoxide Poisoning Hazard | 1 |
| Crash Hazard | 1 |
| Puncture Hazard | 1 |
| Suffocation Hazard | 1 |
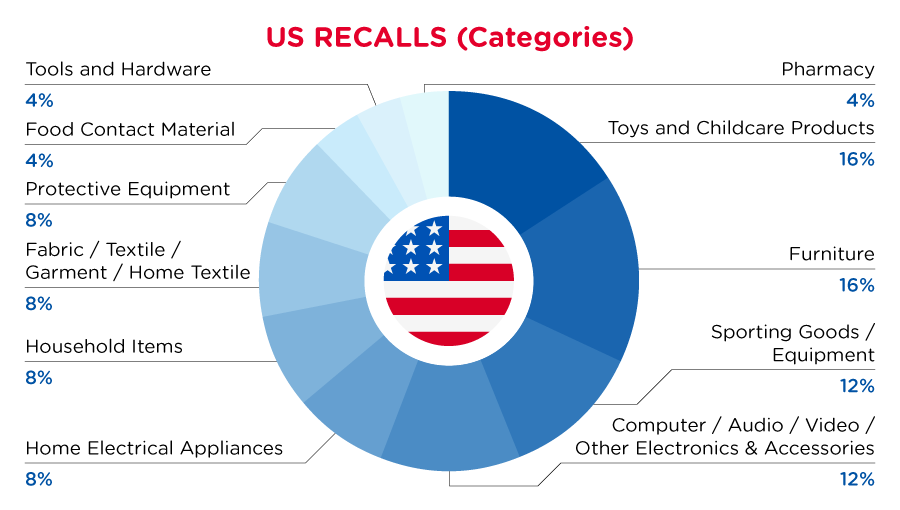
| Product Categories | Frequency |
| Toys and Childcare Products | 4 |
| Furniture | 4 |
| Sporting Goods / Equipment | 3 |
| Computer / Audio / Video / Other Electronics & Accessories | 3 |
| Home Electrical Appliances | 2 |
| Household Items | 2 |
| Fabric / Textile / Garment / Home Textile | 2 |
| Protective Equipment | 2 |
| Food Contact Material | 1 |
| Tools and Hardware | 1 |
| Pharmacy | 1 |
For a complete list click here
In Canada, when hazards are identified in consumer products, they will be recalled and published in the Recalls and Safety Alerts Database on the Health Canada website, which is updated daily. The Canada recalls from 01 June 2023 to 30 June 2023 are summarized below:
Read More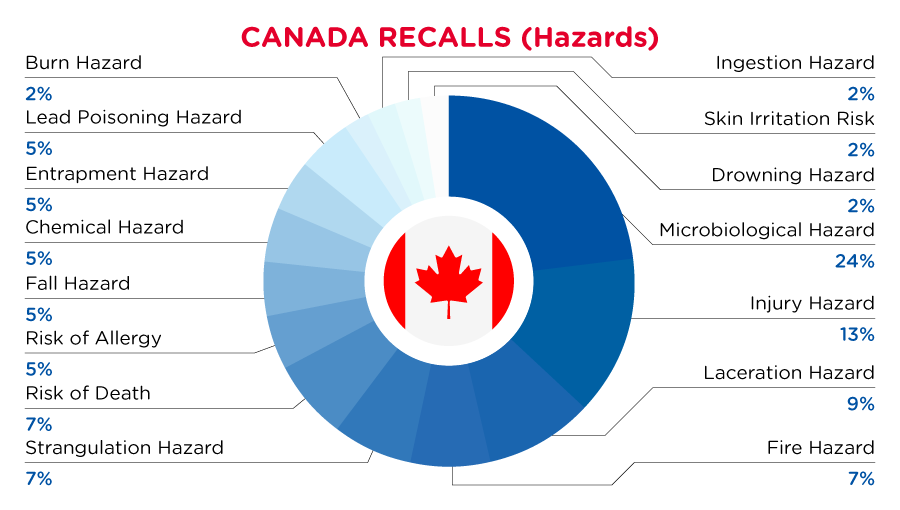
| Hazards | Frequency |
| Microbiological Hazard | 10 |
| Injury Hazard | 6 |
| Laceration Hazard | 4 |
| Fire Hazard | 3 |
| Strangulation Hazard | 3 |
| Risk of Death | 3 |
| Risk of Allergy | 2 |
| Fall Hazard | 2 |
| Chemical Hazard | 2 |
| Entrapment Hazard | 2 |
| Lead Poisoning Hazard | 2 |
| Burn Hazard | 1 |
| Ingestion Hazard | 1 |
| Skin Irritation Risk | 1 |
| Drowning Hazard | 1 |
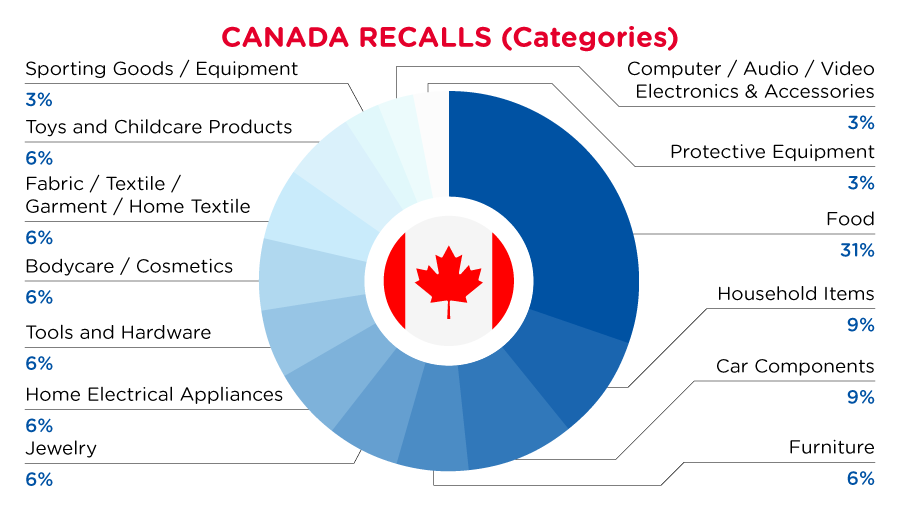
| Product Categories | Frequency |
| Food | 10 |
| Household Items | 3 |
| Car Components | 3 |
| Furniture | 2 |
| Jewelry | 2 |
| Home Electrical Appliances | 2 |
| Tools and Hardware | 2 |
| Bodycare / Cosmetics | 2 |
| Fabric / Textile / Garment / Home Textile | 2 |
| Toys and Childcare Products | 2 |
| Sporting Goods / Equipment | 1 |
| Computer / Audio / Video / Other Electronics & Accessories | 1 |
| Protective Equipment | 1 |
For a complete list click here
In Australia, when hazards are identified in consumer products, they will be recalled and published in the Recalls and Safety Alerts Database on the Australian Competition & Consumer Commission website, which is updated daily. The Australia recalls from 01 June 2023 to 30 June 2023 are summarized below:
Read More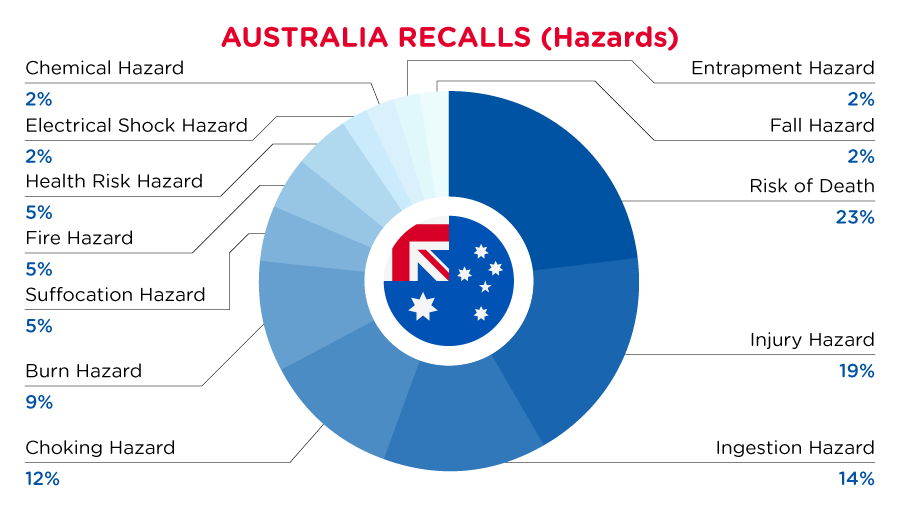
| Hazards | Frequency |
| Risk of Death | 10 |
| Injury Hazard | 8 |
| Ingestion Hazard | 6 |
| Choking Hazard | 5 |
| Burn Hazard | 4 |
| Suffocation Hazard | 2 |
| Fire Hazard | 2 |
| Health Risk Hazard | 2 |
| Electric Shock Hazard | 1 |
| Chemical Hazard | 1 |
| Entrapment Hazard | 1 |
| Fall Hazard | 1 |
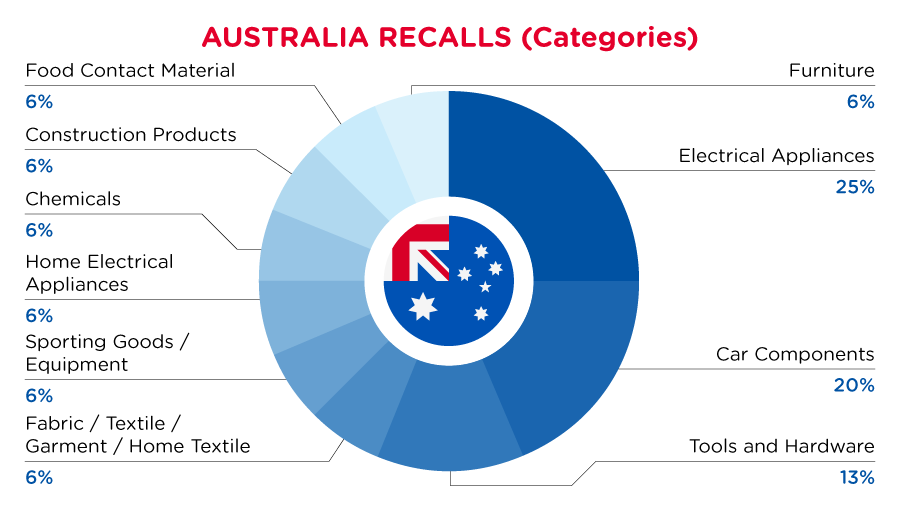
| Product Categories | Frequency |
| Electrical Appliances | 4 |
| Car Components | 3 |
| Tools and Hardware | 2 |
| Fabric / Textile / Garment / Home Textile | 1 |
| Sporting Goods / Equipment | 1 |
| Home Electrical Appliances | 1 |
| Chemicals | 1 |
| Construction Products | 1 |
| Food Contact Material | 1 |
| Furniture | 1 |
For a complete list click here
On 12 July 2023, a new Regulation (EU) 2023/1442 amending Annex I to Food Contact Plastic Regulation (EU) No 10/2011 was published by the European Union.
Read MoreOn 12 July 2023, the European Union (EU) published the 16th amendment (Regulation (EU) 2023/1442 ) to Food Contact Plastic Regulation (EU) No 10/2011. This new amendment includes several changes:
- Deletes two authorized food contact substances: entry 96 wood flour and fibers, untreated, and entry 121 salicylic acid
- Adds five more substances as authorized substances which can be intentionally used in the manufacture of food contact plastic materials and articles
- Requires new limits or group restrictions for the authorized phthalates (DBP, BBP, DEHP and DINP)
- Replaces group restriction to food contact substance entry 793 and entry 822
- Adjusts the restriction of food contact substances entry 1059, entry 1076 and entry 1007
The 16th amendment will enter into force on 1 August 2023. In accordance with Article 2, Transitional measures, of this amendment, “plastic materials and articles complying with Regulation (EU) No 10/2011 as applicable before the entry into force date of this Regulation, which were first placed on the market before 1 February 2025 may remain on the market until the exhaustion of stocks.”
In June 2023, the European Chemical Agency (ECHA) announced the addition of two new chemicals [Diphenyl(2,4,6-trimethylbenzoyl)phosphine oxide (CAS number 75980-60-8), Bis(4-chlorophenyl) sulphone (CAS number 80-07-9)] to the REACH Substances of Very High Concern (SVHC) candidate List.
The REACH SVHC candidate List now has 235 entries.
Read MoreEarlier this year, the European Chemical Agency (ECHA) launched the public consultation for the updates of Substances of Very High Concern (SVHC) chemicals.
On 14 June 2023, ECHA announced the addition of two new chemicals [Diphenyl(2,4,6-trimethylbenzoyl)phosphine oxide (CAS number 75980-60-8), Bis(4-chlorophenyl) sulphone (CAS number 80-07-9)] to the REACH SVHC Candidate List.
Hazards associated with these chemicals:
- Diphenyl(2,4,6-trimethylbenzoyl)phosphine oxide is toxic for reproduction,
- Bis(4-chlorophenyl) sulphone has very persistent and very bioaccumulative (vPvB) hazardous properties.
Details of these two new SVHCs are listed in below table.
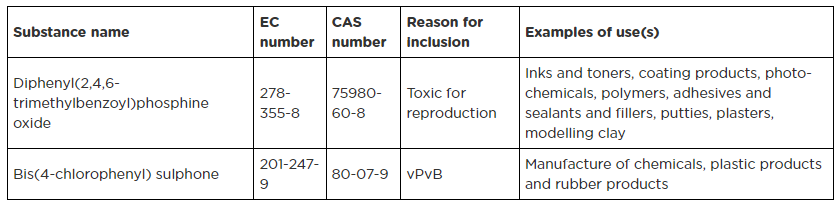
Hence, the current REACH SVHC Candidate List now has 235 entries.
(Some SVHCs are restricted in “groups” of chemicals, the total number of impacted chemicals is more than 235 species.)
In Europe, when hazards are identified in non-food consumer products, the products will be recalled and published in the Safety Gate system, which is updated weekly. The European recalls from 01 June 2023 to 30 June 2023 are summarized below:
Read More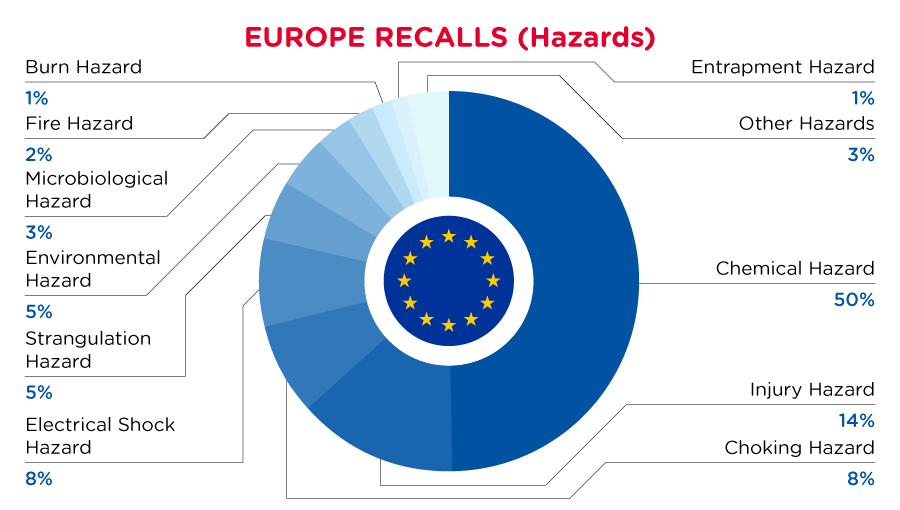
| Hazards | Frequency |
| Chemical Hazard | 132 |
| Injury Hazard | 36 |
| Choking Hazard | 21 |
| Electric Shock Hazard | 20 |
| Strangulation Hazard | 13 |
| Environmental Hazard | 12 |
| Microbiological Hazard | 8 |
| Fire Hazard | 6 |
| Burn Hazard | 4 |
| Entrapment Hazard | 4 |
| Other Hazards* | 9 |
*Other Hazards include Damage to Sight, Health Risk Hazard, Cut Hazard and Damage to Hearing with a frequency of less than 4.
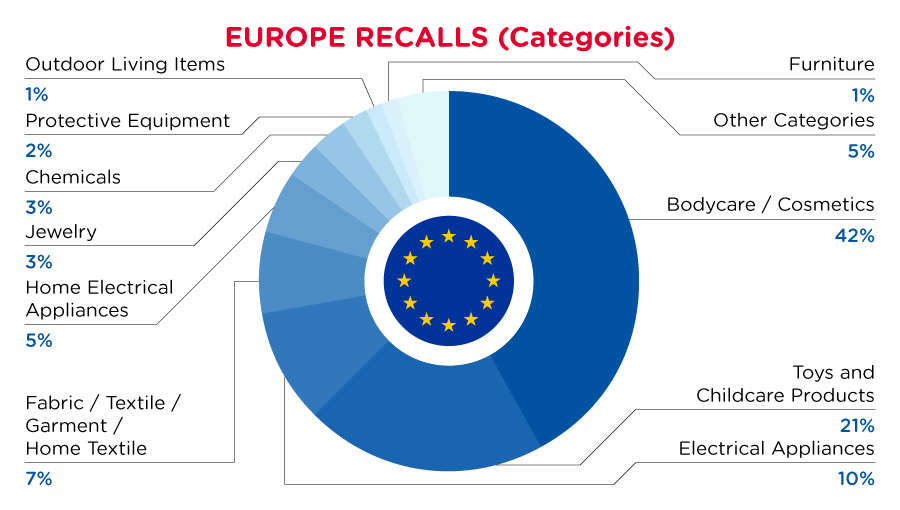
| Product Categories | Frequency |
| Bodycare / Cosmetics | 96 |
| Toys and Childcare Products | 47 |
| Electrical Appliances | 22 |
| Fabric / Textile / Garment / Home Textile | 16 |
| Home Electrical Appliances | 12 |
| Jewelry | 7 |
| Chemicals | 7 |
| Protective Equipment | 5 |
| Outdoor Living Items | 3 |
| Furniture | 3 |
| Other Categories* | 10 |
*Other Categories include Sporting Goods / Equipment, Machinery, Household Items, Footwear, Accessories, Construction Products and Pet Items with a frequency of less than 3.
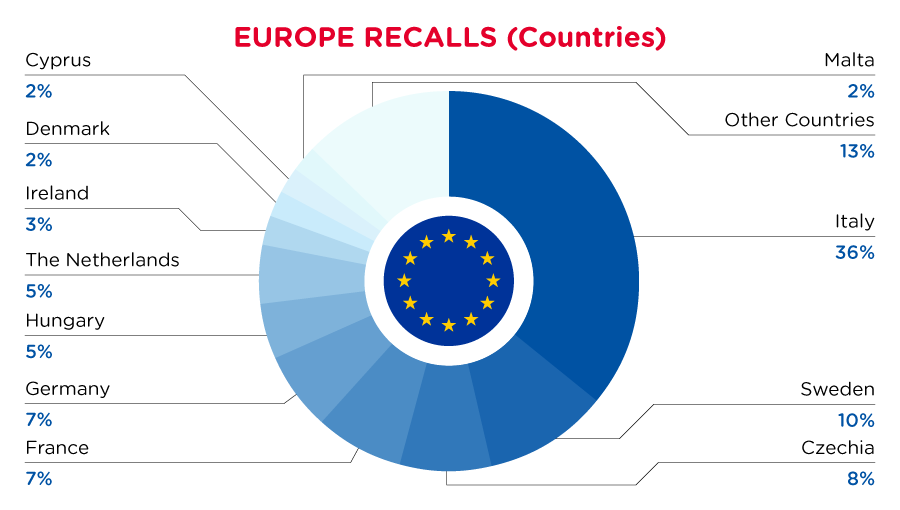
| Notifying Country | Frequency |
| Italy | 82 |
| Sweden | 24 |
| Czechia | 18 |
| France | 17 |
| Germany | 15 |
| Hungary | 11 |
| The Netherlands | 11 |
| Ireland | 6 |
| Denmark | 5 |
| Cyprus | 5 |
| Malta | 5 |
| Other Countries* | 29 |
*Other Countries include Poland, Finland, Croatia, Lithuania, Belgium, Estonia, Bulgaria, Slovakia, Austria, Iceland, Spain and Slovenia with a frequency of less than 5.
For a complete list click here
On 26 December 2022, China Cybersecurity Review Technology and Certification Center (CCRC) published the Implementation Rules CCRC-C09-01: 2022 on the Compulsory Certification of Information Technology Equipment.
Read MoreChina Cybersecurity Review Technology and Certification Center (CCRC) published Implementation Rules CCRC-C09-01: 2022 on the Compulsory Certification of Information Technology Equipment on 26 December 2022.
These implementation rules involve nine product categories:
- Microcomputers (including self-service terminals)
- Portable Computers
- Display Devices Connected to Computers
- Printing Devices Connected to Computers
- Multi-purpose Printer / Copiers
- Scanners
- Power Supplies
- Servers
- Cash Registers
The rules apply to products in the above categories which are supplied power (directly or indirectly) by a power supply over 36 V d.c. or 36 V a.c. rms. The compliance certification requirements must be according to Safety Standard GB 4943.1 (for AV and IT equipment) and EMC Standards GB/T 9254.1 (for emissions requirements for IT equipment, multimedia equipment and receivers) and GB 17625.1 (for electromagnetic capability - limits – Part 1 for harmonic current emissions) with the exception of Cash Registers in which GB 17625.1 is not applicable.
Four certification modes and three principles of adoption were classified in these implementation rules based on the type of protection against electric shock of product and enterprise categories. Classification of certification unit such as applicant, product category(ies), construction, schematics, etc. are also listed in the implementation rules.
Specific for applicants, the procedures on how to apply for certification, including the necessary materials and documentation were established, while the specifics on how to review and arrange certification procedures were set up for the CCRC.
Most notably, the implementation rules give detailed information about the type of testing protocols, sampling requirements, test items, implementation of specific testing, initial factory inspections, test result evaluations, completion of testing reports, and issuance of the certification, surveillance, and tracking obligations after issuing the certificate.
The validity of the certificate is five years. In the event of any changes to the products, the certificate holder must submit a modification application of such changes and ensure that the certified products are consistent with the type of test report corresponding to the product.
The Chinese Compulsory Certificate (CCC) marking should be affixed on the certified product body in a conspicuous manner before leaving the factory for product manufactured domestically, or before importing for product manufactured overseas.
The implementation rules will enter into force on 1 August 2023.
In China, when hazards are identified in consumer products, they will be recalled and published in the SAMR Defective Product Administrative Centre, which is updated daily. The China recalls from 01 June 2023 to 30 June 2023 are summarized below:
Read More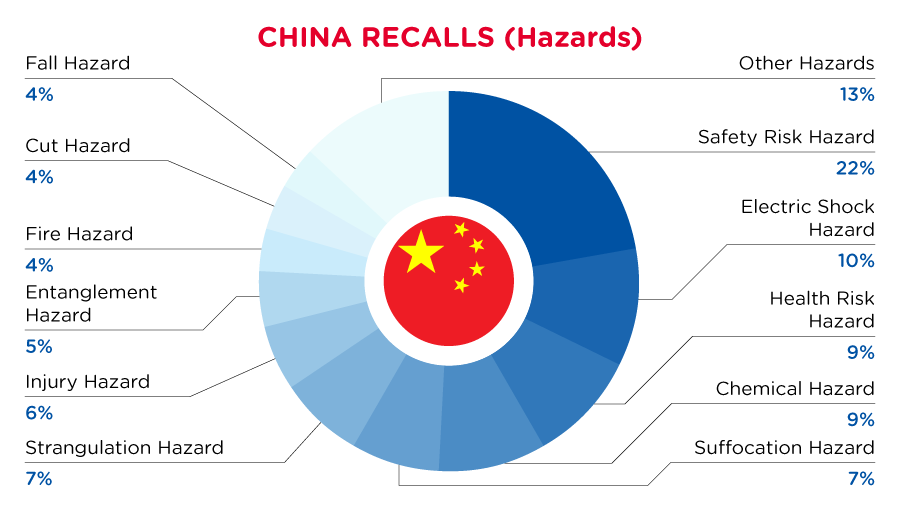
| Hazards | Frequency |
| Safety Risk Hazard | 24 |
| Electric Shock Hazard | 11 |
| Health Risk Hazard | 10 |
| Chemical Hazard | 10 |
| Suffocation Hazard | 8 |
| Strangulation Hazard | 8 |
| Injury Hazard | 6 |
| Entanglement Hazard | 5 |
| Fire Hazard | 4 |
| Cut Hazard | 4 |
| Fall Hazard | 4 |
| Other Hazards* | 14 |
*Other Hazards include Lead Poisoning Hazard, Eye Irritation Risk, Swallowing Risk, Crushing Hazard, Risk of Allergy, Choking Hazard, Explosion Hazard and Ingestion Hazard with a frequency of less than 4.
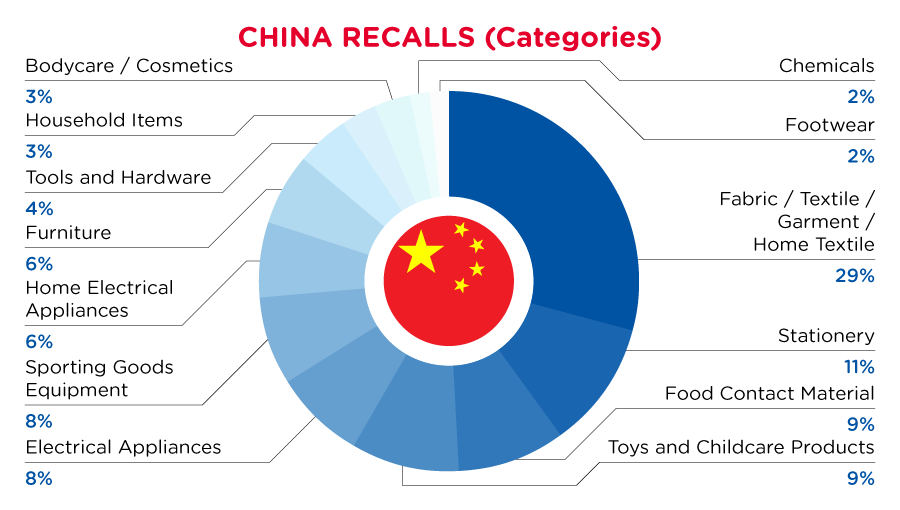
| Product Categories | Frequency |
| Fabric / Textile / Garment / Home Textile | 19 |
| Stationery | 7 |
| Food Contact Material | 6 |
| Toys and Childcare Products | 6 |
| Electrical Appliances | 5 |
| Sporting Goods / Equipment | 5 |
| Home Electrical Appliances | 4 |
| Furniture | 4 |
| Tools and Hardware | 3 |
| Household Items | 2 |
| Bodycare / Cosmetics | 2 |
| Chemicals | 1 |
| Footwear | 1 |
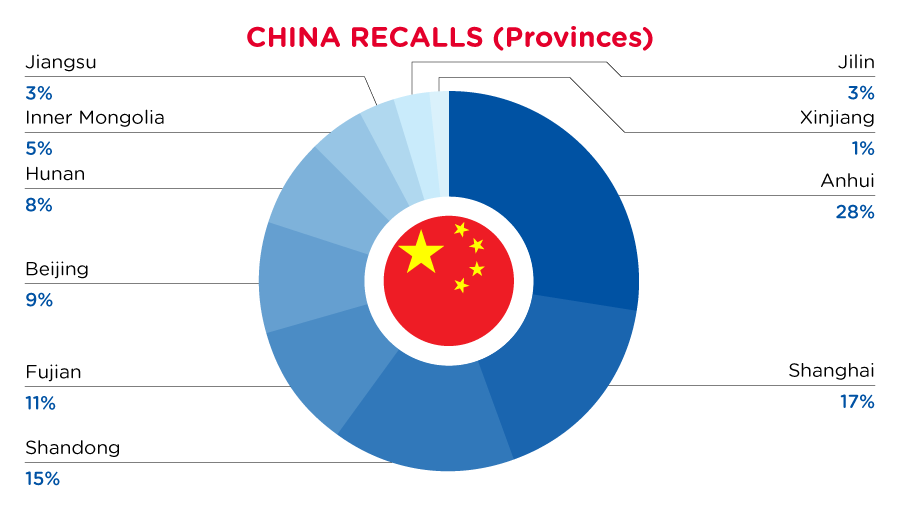
| Provinces | Frequency |
| Anhui | 18 |
| Shanghai | 11 |
| Shandong | 10 |
| Fujian | 7 |
| Beijing | 6 |
| Hunan | 5 |
| Inner Mongolia | 3 |
| Jiangsu | 2 |
| Jilin | 2 |
| Xinjiang | 1 |
For a complete list click here
CẦN THÊM THÔNG TIN?
Thank you - your inquiry has been sent.
We will come back to you shortly.
Back
Subscribe to our Regulatory Updates
Thank you for subscribing.
Contact Us By Phone
+86 755 2223 9888
Sales:+86 755 2223 9086
Thử Nghiệm Trong Phòng Thí Nghiệm:+86 577 6160 3663
Contact a local office
For any questions you may have:
+1 888 264 8988
| This site is protected by copyright and trademark laws under US and international law. |
| QIMA © 2025 |
|
ClientID:; Client:; Affiliate:;
|

