Saudi Food and Drug Authority (SFDA) Certification for Cosmetics
SFDA Announcement
From October 3rd 2021, all importers must request a Certificate of Compliance for their cosmetics shipment via FASEH: https://faseh.sfda.gov.sa/, and once the request has been received, reviewed and assessed by QIMA, we will print and upload the certificate as soon as compliance is verified.
Saudi Food and Drug Authority (SFDA) is primarily responsible for regulating, overseeing, and monitoring food, cosmetics, drugs, and medical devices, as well as establishing mandatory requirements for regulated products. Food, drugs, medical devices, cosmetics, pesticides, and feed are all regulated by SFDA.
It is mandatory for cosmetic products to comply with the SFDA GSO 1943 regulation on Cosmetics and Personal Care products. A list of prohibited substances as well as approved preservatives, UV filters, and colorants is also included in the annexes. Additionally, importers need to ensure that products adhere to requirements published in Circulars issued by the SFDA.
The SFDA defines cosmetic products as those that are intended to be placed into contact with the external parts of the human body such as the skin, hair system, nails, lips, including teeth and mucous membranes of the mouth. Products that are intended for internal uses are not included in scope.
QIMA, a leading provider of quality control and supply chain compliance solutions, announced in April 2022 that it has been approved as a Notified Body by the Saudi Food and Drug Authority (SFDA) to certify cosmetic products being imported or sold in the Kingdom of Saudi Arabia. QIMA is equipped to provide all additional necessary services to help exporters secure product entry into Saudi Arabia including:
- Product testing provided by QIMA’s global network of ISO 17025 accredited laboratories for products that lack a test report or have incomplete testing.
- Product registration services for eCosma.
- Risk Assessment for importers or manufacturers unsure of the risk assessment requirement.
- Label review to help brands avoid delays during the conformity assessment process.
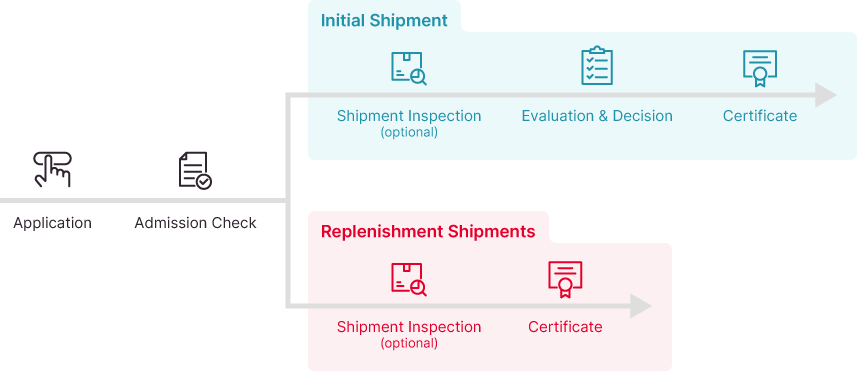
Certification Process