September 2020 Regulatory Update
On July 31, 2020, the U.S. Consumer Product Safety Commission (CPSC) published a final rule to delay the effective date of the CPSC's mandatory standard for hand-held infant carriers, due to the COVID-19 pandemic.
View Story Read MoreOn May 20, 2020, the U.S. Consumer Product Safety Commission (CPSC) published a direct final rule (DFR) (16 CFR 1225) to revise the mandatory standard for hand-held infant carriers to incorporate by reference the most recent version of the applicable ASTM standard, ASTM F2050-19 Standard Consumer Safety Specification for Hand-Held Infant Carriers. The final rule was originally set to become effective on August 3, 2020, unless the Commission received a significant adverse comment by June 19, 2020.
Since Commission approval of the DFR in April 2020, Executive Order (E.O.) 13924, “Regulatory Relief to Support Economic Recovery,” was issued on May 19, 2020. E.O. 13924 encourages federal agencies to address the economic consequences of COVID-19 “by rescinding, modifying, waiving, or providing exemptions from regulations and other requirements that may inhibit economic recovery, consistent with applicable law and with the protection of the public health and safety.”
The effective date for the direct final rule published on May 20, 2020, at 85 FR 30605, is delayed from August 3, 2020, until January 1, 2021.
Contact: Vivian Chan (Technical Consultant)
Phone: (852) 3185 8052
Email: regulatoryupdates@qima.com
The State of Vermont Department of Health has amended the Chemicals of High Concern in Children’s Products Rule on September 1, 2020, and this change will take effect immediately.
View Story Read MoreThe amendments, excerpted below, include the revision of reporting deadlines and the addition to the CHCC list by expanding "formaldehyde" to include formaldehyde-related compounds.
8.0 Reporting Years and Periods
8.1 On or before January 31, 2022, and annually thereafter, a manufacturer of a children’s product offered for sale or distribution in Vermont shall submit to the Department the notice described in Section 6.0 of this rule.
9.0 Reporting Between Annual Reporting Periods
9.1 On or before July 31 of every year, a manufacturer of a children’s products shall report all products introduced for sale or distribution in Vermont between January 31 and July 31 of that year in accordance with Section 6.0 of this rule.
5.0 Chemicals of High Concern to Children
The following chemicals are designated as chemicals of high concern to children: (1) Formaldehyde and substances that are intentionally added to release formaldehyde, including 5-Bromo-5-nitro-1,3-dioxane, Bronopol, Diazolidinyl urea, DMDM hydantoin, Imidazolidinyl urea, Methanol, (phenylmethoxy), Methenamine, Quaternium-15, and Sodium N-(hydroxymethyl)glycinate.
Additional information on reporting is available on the Department's website.
Contact: Andy Choi (Senior Manager)
Phone: (852) 3185 8045
Email: regulatoryupdates@qima.com
In the US, when hazards are identified in consumer products, they will be recalled and published in the Consumer Product Safety Commission (CPSC) Recent Recalls on the CPSC website, which is updated daily. The US recalls from April 1, 2019 to September 16, 2020 are summarized below:
View Story Read More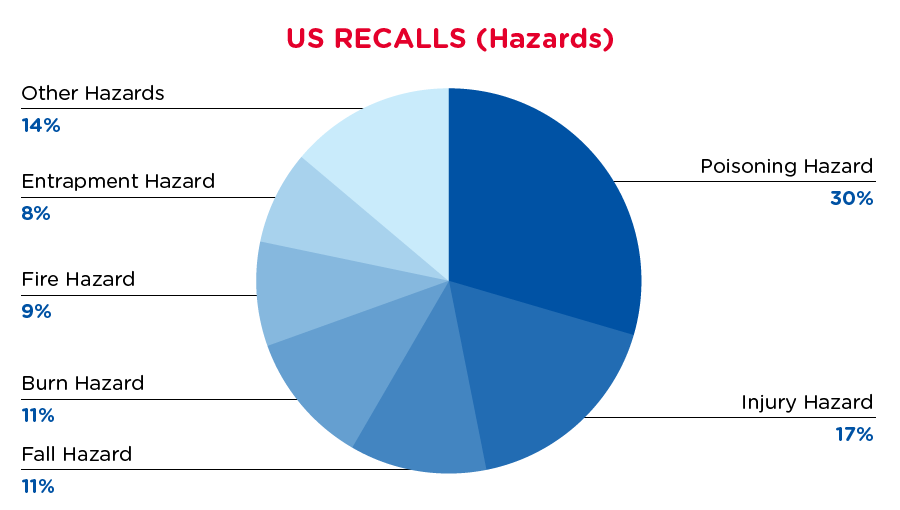
| Hazards | Frequency |
| Poisoning Hazard | 34 |
| Injury Hazard | 20 |
| Fall Hazard | 13 |
| Burn Hazard | 13 |
| Fire Hazard | 10 |
| Entrapment Hazard | 9 |
| Other Hazards* | 16 |
*Other Hazards include, Crash Hazard, Death Reports, Entrapment Hazard, Laceration Hazard, Shock Hazard, Strangulation Hazard, Skin Irritation Hazard, Strangulation Hazard and Choking Hazard with a frequency of less than 7.
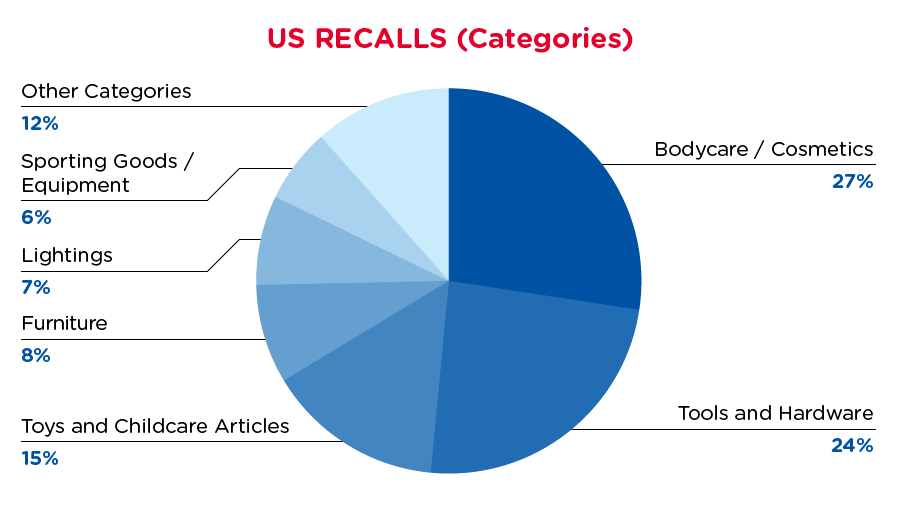
| Product Categories | Frequency |
| Bodycare / Cosmetics | 26 |
| Tools and Hardware | 23 |
| Toys and Childcare Articles | 14 |
| Furniture | 8 |
| Lightings | 7 |
| Sporting Goods / Equipment | 6 |
| Other Categories^ | 11 |
^Other Categories include Food Contact Material, Home Electrical Appliances, Fabric / Textile / Garment / Home Textiles with a frequency of less than 7.
For a complete list click here
On August 31, 2020, The Competition Bureau is inviting feedback on proposed changes to the Textile Labelling and Advertising Regulations (TLAR), which relates to upholstered and stuffed articles. The proposed changes are intended to simplify the labelling of upholstered and stuffed articles and provide greater clarity for businesses.
View Story Read MoreThis document outlines proposed changes to the Textile Labelling and Advertising Regulations (TLAR) that relate to upholstered and stuffed articles.
1. Exemption for textile articles labelled under provincial regulations
Proposal: The provincial exemption under section 10 of the TLAR would be simplified to exempt only fibre-related label representations that are required by provincial law. This expansion of the section to all textiles will avoid the need for future amendments to section 10 should a specific province create new labelling requirements.
2. Labelling of textiles “used for warmth” and “textiles not used for warmth”
Proposal: The TLAR labelling requirements under section 37 (upholstered and stuffed articles used for warmth) and subsection 38(1) (upholstered and stuffed articles not used for warmth) would be harmonized and combined into one section.
3. Labelling exemption for certain filled and stuffed textile articles
Proposal: Repealing subsection 38(2) of the TLAR, which currently exempts dealers from providing information on a label regarding the fibre composition of filling or stuffing used in upholstered furniture, mattresses, box springs, cushions, chair pads, potholders, oven mitts, place mats, and mattress protectors (subsection 38(2) articles). Under this proposal, dealers would be required to provide information on a label regarding the filling or stuffing for all subsection 38(2) articles.
4. Filled and stuffed textile articles produced prior to January 1, 2021
Proposal: The TLAR would include a new section that allows dealers to rely on the subsection 38(2) exemption for articles produced before January 1, 2021. Dealers of subsection 38(2) articles made after that date would be required to disclose the fibre composition of the filling or stuffing used in the article.
5. Textile Articles Subject to the TLA and TLAR
Proposal: The Bureau proposes amending Schedule I (3) of the TLAR to prescribe all textile components of subsection 38(2) articles. The previous Schedule I (3) only was related to the outer covering of the article.
6. Consumer Textile Articles containing filling or stuffing
Proposal: This proposal would amend the current wording of Schedule II (5) of the TLAR to clarify that the section is limited to consumer textile articles where the only textile fibre(s) present are filling or stuffing (e.g. a bag of stuffing at a craft store).
7. Non-permanent labels
Proposal: This proposal would amend Schedule III (3) to allow dealers to use non-permanent labels to disclose all textile components of subsection 38(2) articles.
The comment period runs through October 29, 2020.
Contact: David Zhao (Technical Consultant)
Phone: (571) 8999 7142
Email: regulatoryupdates@qima.com
On August 27 2020, the ACCC announced an updated MANDATORY safety standard for toys containing magnets had been issued. These requirements are intended to reduce the risk of serious injury or death to children from swallowing small hazardous magnets.
The new standard provides a transitional period of 12 months. Starting August 29 2021, suppliers must meet the requirements of the Consumer Goods (Toys Containing Magnets) Safety Standard 2020.
The Consumer Goods (Toys Containing Magnets) Safety Standard 2020 sets design and construction requirements to prevent a child from gaining access to small hazardous magnets. The standard limits the supply of toys with loose small high- powered magnets to only magnetic/electrical experimental sets intended for children eight years of age and over.
The mandatory standard applies to toys containing magnets that are:
- Designed or clearly intended for use in play by a child under 14 years of age
- With one or more magnets or magnetic components
The mandatory standard excludes:
- Sporting goods
- Camping goods
- Bicycles
- Home and public playground equipment
- Trampolines
- Electronic game units
- Models powered by combustion or steam engines
- Fashion jewellery
Design and construction
Toys containing magnets must comply with the relevant sections of one of the following standards:
- Australian/New Zealand Standard AS/NZS 8124.1:2019 - Safety of Toys - Part 1: Safety Aspects Related to Mechanical and Physical Properties
- European Standard EN71-1:2014+A1:2018 Safety of toys – Part 1: Mechanical and physical properties
- International Standard ISO 8124.1:2018 Safety of Toys - Part 1: Safety Aspects Related to Mechanical and Physical Properties
- US Standard ASTM F963-17 Standard consumer Safety Specification for Toy Safety
Warning requirements
The mandatory standard prescribes warning requirements to accompany magnetic/electrical experimental sets intended for children eight years of age and over with loose small high-powered magnets. These warnings alert parents and caregivers to the risk of swallowing small hazardous magnets.
Contact: Vivian Chan (Technical Consultant)
Phone: (852) 3185 8052
Email: regulatoryupdates@qima.com
On September 8 2020, the European Commission (EC) notified the World Trade Organisation (WTO) of the draft Directive amending Appendix C to Annex II to the Toy Safety Directive 2009/48/EC in order to add new limits for aniline in certain toy materials. The restrictions are proposed to become effective in Q3, 2022.
View Story Read MoreThe Commission proposed to add Aniline (CAS no.: 62-53-3) as an entry in Appendix C to Annex II to the Toy Safety Directive. Aniline (CAS no.: 62-53-3), which has been classified as carcinogenic category 2 and mutagenic category 2, is considered a non-threshold carcinogen, implying that it may cause cancer at even the slightest level of exposure. Based on various scientific studies, evidence of its presence in toys and experts’ discussions, the Commission now proposes to regulate aniline in toys for use by children under 3 years of age or in other mouthable toys as follows:
| Substance | CAS No | Limit value |
| Aniline | 62-53-3 | 30 mg/kg after reductive cleavage in textile toy material and leather toy material 10 mg/kg as free aniline in finger paints 30 mg/kg after reductive cleavage in finger paints |
Contact: Andy Choi (Senior Manager)
Phone: (852) 3185 8045
Email: regulatoryupdates@qima.com
On September 3, 2020, the European Union (EU) published Regulation (EU) 2020/1245 to amend Regulation (EU) 10/2011 on plastic materials and articles intended to come into contact with food.
View Story Read MoreIn the new rule, the key amendments to the plastic food contact requirements are summarized below:
- New specific migration limits in specific migration of heavy metals
Test item Specific migration limit Arsenic Not Detected (Detection limit: 0.01 mg/kg) Cadmium Not Detected (Detection limit: 0.002 mg/kg) Chromium Not Detected
(Detection limit:
• 3.6 mg/kg if there is proof to exclude the presence of chromium (VI),
• 0.01 mg/kg for other cases)Europium Sum and individual of lanthanide substances (europium, gadolinium, lanthanum and terbium) shall not exceed 0.05 mg/kg Gadolinium Lanthanum Terbium Lead Not Detected (Detection limit: 0.01 mg/kg) Mercury Not Detected (Detection limit: 0.01 mg/kg) - Updated Specific Migration of Primary Aromatic Amines (PAAs) requirements
Test item Specific migration limit Each PAA listed in entry 43 to Appendix 8 of Annex XVII to REACH Regulation (EC) No 1907/2006, and no migration limit is specified in Table 1 of Annex I to Regulation (EU) 10/2011 ≤ 0.002 mg/kg Sum of PAAs not listed in entry 43 to Appendix 8 of Annex XVII to REACH Regulation, and no migration limit is specified in Annex I to Regulation (EU) 10/2011 ≤ 0.01 mg/kg - New overall migration testing condition, OM0 (30 min at 40 °C) for any food contact at cold or ambient temperatures and for a short duration (≤ 30 minutes).
- Introduced reflux testing condition to overall migration condition OM4 in the case that testing at 100°C is technically unavailable.
- Updated the specification of testing repeated use articles.
- Minor update in the union list of authorized substances.
A two year transition period is introduced according to the new rule. Plastic materials and articles which are placed on the market before March 23, 2021, may continue to be sold until September 23, 2022 and remain on the market until the exhaustion of stocks.
Contact: Andy Choi (Senior Manager)
Phone: (852) 3185 8045
Email: regulatoryupdates@qima.com
On August 11, 2020, the France authority issued Order of August 5, 2020 to update the requirements of food contact rubber materials and articles, and pacifiers for infants and young children. The new Order will come into force on July 1, 2021 to replace the Decree of November 9, 1994.
View Story Read MoreAccording to the new Order, the key amendments to the rubber food contact regulation are summarized as below.
- Updated the definition of rubber materials that the Order only applies to natural rubber and vulcanized thermoplastic elastomers but not silicone elastomer
- Updated the list of authorized substances and the specific restrictions on certain listed substances
- Updated the specification of overall migration limits
Category of product Limit - All articles in scope
- Pacifiers for infants and young children
- Gaskets, valves and valve elements where the ratio between the surface in contact with food and the volume is not known or specified
≤ 10 mg/dm2 - Food contact materials and articles for infants and young children
- Bottle nipples and teats
- Gaskets, valves and valve elements where the ratio between the surface in contact with food and the volume is known or specified
≤ 60 mg/kg - Introduced a new Declaration of Compliance (DoC) to demonstrate the compliance with the Order
According to the Order, products which are placed on the market before July 1, 2021 can continue to be placed on the market until the exhaustion of stocks.
Contact: Andy Choi (Senior Manager)
Phone: (852) 3185 8045
Email: regulatoryupdates@qima.com

